Russula succinea G.J. Li & C.Y. Deng, sp. nov.2021
Fungal Names: FN 570760
Holotype: China, Guizhou Province, Weining Yi, Hui, and Miao Autonomous County, Caohai National Nature Reserve, 26°53'N, 104°12'E, alt. 2183 m, on the ground in coniferous forest, 15 July 2017, C.Y. Deng CH2017071509 (HGAS-MF 009904, Holotype). GenBank accession: MN649188 (ITS).
Morphological description
Sexual morph: Basidiomata small to medium sized. Pileus 32–54 mm in diam., ini tially hemispherical, then plano-convex, flat when mature, often slightly depressed at centre, strongly viscid when wet, yellowish-brown tinged, pale brownish tinged, often intermixed with greyish-yellow fringe, Hazal (XIV11′k), Russet (XV13′k), Cinnamon Brown (XV15′k) to Tawny (XV15′) at centre, rarely with Liver Brown (XIV17′m), Pe can Brown (XXVIII13′′i) or Rood’s Brown (XXVIII11′′k) when old and dry; margin subacute to acute, straight, rarely split or inward-turned, tuberculate-striate 14–25 mm from the edge inwards, peeling 1/3–1/2 towards the centre, pale yellowish tinged, first Deep Colonial Buff (XXX21′′b), Honey Yellow (XXX19′′) to Light Ochraceous Salmon (XV13′d), then Light Cadmium (IV19), Maize Yellow (III19f) when mature. Lamellae adnate, 3–6 mm in height at the halfway point of pileus radius, brittle, often forked near the stipe and pileus edge, interveined, pale cream-coloured, first White (LIII), Cream Colour (XVI19′f) when mature, sometimes stained with Martius Yellow (III23f) to Baryta Yellow (IV21f); edge entire, narrowing towards the pileus margin, 13–22 piec es per cm in the edge; lamellulae absent. Stipe slightly subcentral, rarely central, 4.2 8.3 × 1.5–2.2 cm, subclavate to subcylindrical, often narrowing towards the base, rarely slightly curved, smooth when young, rugulose longitudinally in age, dry, Cream Colour (XVI19′f), staining Sudan Brown (III15k) to Orange-Citrine (IV19k) when bruised, Tawny Olive (XXIX17′′i), Sayal Brown (XXIX15′′) to Isabella Colour (XXX19′′i) at base, initially solid, turning hollow in age. Context White (LIII), unchanging when bruised or touched, 3–5 mm thick at the centre of pileus, fragile, taste first slightly acrid, mild when mature, odour indistinct. Spore print pale cream (Romagnesi IIc–IId).
Basidiospores [350/14/7] (5.8–) 6.1–7.8 (–8.3) × (4.9–) 5.2–6.8 (–7.3) μm, Q = (1.00–) 1.03–1.30 (–1.33) (Q = 1.17 ± 0.08), 7.0 × 6.0 μm on average, globose, subglobose to broadly ellipsoid, rarely ellipsoid, composed of verrucous to subcylindri cal amyloid warts 0.8–1.2 μm in height, often linked as short to long crests and ridges, forming an incomplete reticulum, rarely intermixed with isolated warts; suprahilar spot distinct, but not amyloid. Basidia 44–66 × 10–12 μm, mostly 4-spored, clavate to subcylindrical; sterigmata 4–6 μm in length, straight to tortuous. Hymenial cystidia moderately numerous, ca. 700–1300/mm2, 71–88 × 9–15 μm, fusiform, some times cylindrical, thin-walled, apex obtuse, rarely mucronate, projecting 20–40 μm beyond the hymenium, contents granular to crystalline, partly dense, blackish-grey in SV. Pileipellis two-layered, distinctly delimited from the underlying context. The upper suprapellis (70–130 μm thick) in pileus centre an ixotrichoderm, composed of ascending to erect hyphae 4–7 μm in width, septate, cylindrical, often slightly inflated, acid-resistant encrustations absent, terminal cells sometimes narrowing towards the apex, subapical cells cylindrical, not branched; suprapellis a trichoderm in pileus mar gin, composed of repent, slender, cylindrical, hyaline hyphae 3–5 μm in width, acid resistant encrustations absent. Pileocystidia abundant, long, cylindrical, often septate, 4–10 μm in width, apex obtuse, contents granulate, dense, blackish-grey in SV. The lower layer subpellis (50–90 μm thick) composed of loosely interwoven, mostly repent, cylindrical, septate hyaline hyphae often inflated, 2–7 μm in width. Clamp connections not observed in all parts.
Asexual morph: Undetermined
Culture characteristics:
Habitat: Single to scattered on yellow brown soil in coniferous forest dominated by Pinus armandii, P. massoniana and P. yunnanensis at 1200–2200 m altitude.
Distribution: China (Guizhou and Jiangxi).
GenBank Accession:
MN649196
MN649188
MN649198
MN649190
MN258682
Notes: This new species is reminiscent of R. foetentula, R. obscuricolor and R. rufobasalis because of the reddish-brown or burnt sienna colour at the stipe base (Peck 1907; Song et al. 2018). The following characters are helpful for differentiat ing these two species from R. succinea: R. foetentula has lower basidiospore orna mentations 0.5–0.9 μm connected by occasional to rare line connections, hymenial cystidia with mucronate-appendiculate apices 2–7 μm long, pileocystidium apex often constricted to 1–2.5 μm in width; North American distribution (Peck 1907; Adamčík et al. 2013); R. obscuricolor has darker brown to chocolate brown tinges at pileus centre, bitter to pungent taste of context and shorter hymenial cystidia (pleurocystidia 30–65 × 6–9 μm, cheilocystidia 23–33 × 5–7 μm) (Das et al. 2017); R. rufobasalis has bright reddish tinge at stipe base, basidiospore ornamentations 0.3–0.8 μm in height and frequently thick-walled, narrower terminal cells 2–5 μm in width (Song et al. 2018).
For those Asian sect. Ingratae members that have similar pileus tinges, R. ahmadii can be distinguished from R. succinea by lower basidiospore ornamentations up to 0.3 μm, shorter basidia (29–) 29.7–38.9 (–40.1) × (9.2–) 9.4–11.3 (–11.8) μm and pileipellis a cutis (Jabeen et al. 2017); R. arunii differs from the new species in the orange tinge intermixed on pileus surfaces, white spore print, narrow pileocystidia 3–4 μm in width and a habit of broad-leaved Pterigota alata forest (Crous et al. 2017); R. catillus differs in that basidiospore ornamentation is composed of mostly isolated, verrucous to conical warts, absence of pileocystidia in pileipellis and a habitat of oak hardwood forest (Lee et al. 2017); R. indocatillus can be differentiated from the new species for white spore print, shorter basidia 34–40 × 9–11 μm and capitate hymenial cystidium apex (Ghosh et al. 2020); R. natarajanii differs in having light to medium brown spots at the pileus periphery, shorter basidia 28–35 × 7.5–9 μm and a habitat of Quercus forest (Das et al. 2006); R. pseudocatillus differs in the presence of lamel lula, basidiospores ornamented with isolated warts never forming a reticulum and a habitat of broad-leaved evergreen forest (Yuan et al. 2019); R. pseudopectinatoides can be distinguished from the new species in having hymenial cystidia with moniliform or capitate apex, larger basidiospores up to 9 μm in diam. and absence of pileocystidia (Li et al. 2015b); R. straminella differs in its shorter basidia and hymenial cystidia, often thick-walled terminal cells in pileipellis of pileus margin (Figs 6 and 7); R. gelatinosa, R. punctipes, R. seneicis, R. subpunctipes and R. tsokae differ from R. succinea in their larger basidiospores (9 μm in diam.) with high ornamentation up to 2 μm in height (Khatua et al. 2015; Lee et al. 2017; Song et al. 2018; 2020).
Reference: Li, G. J., Li, S. M., Buyck, B., Zhao, S. Y., **e, X. J., Shi, L. Y., ... & Li, M. (2021). Three new Russula species in sect. Ingratae (Russulales, Basidiomycota) from southern China. MycoKeys, 84, 103.
Figure 2. Basidiomata E–F Russula succinea. Bars: 10 mm. 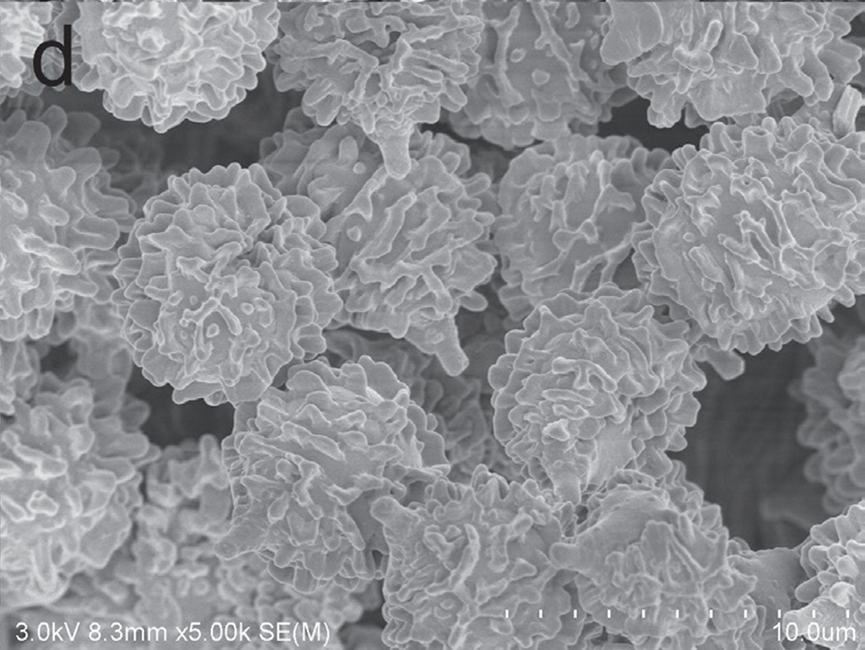
Figure 3. SEM photo of basidiospores D Russula succinea. 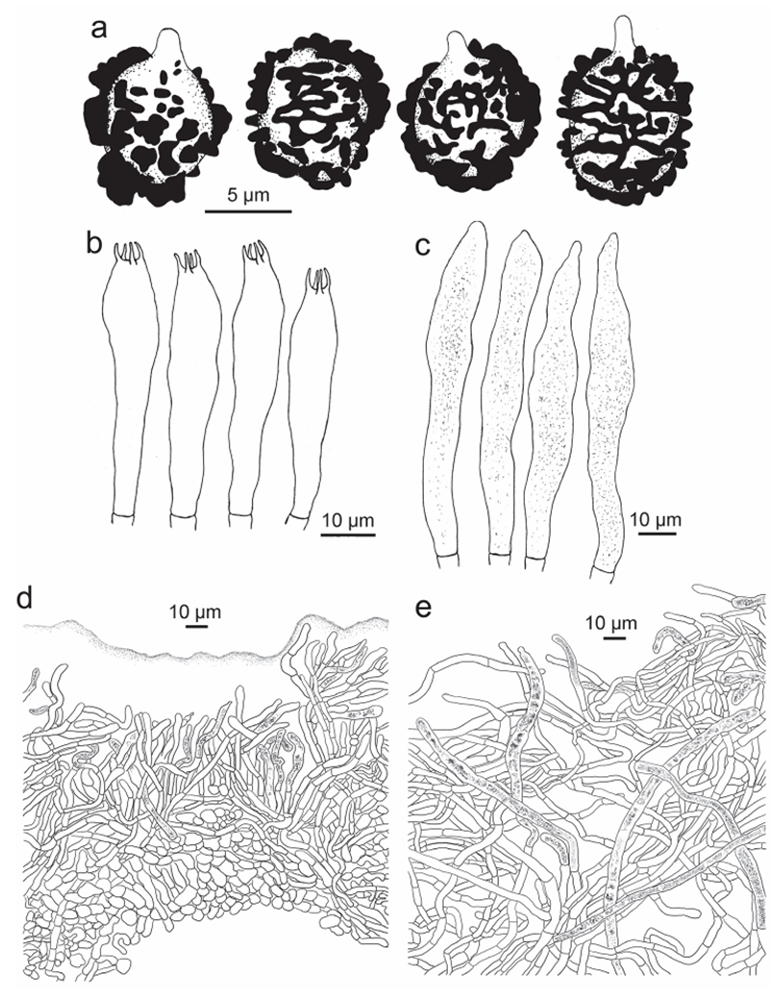
Figure 10. Russula succinea, holotype A basidiospores B basidia C hymenial cystidia D suprapellis par tial subpellis in pileus centre E suprapellis in pileus margin. 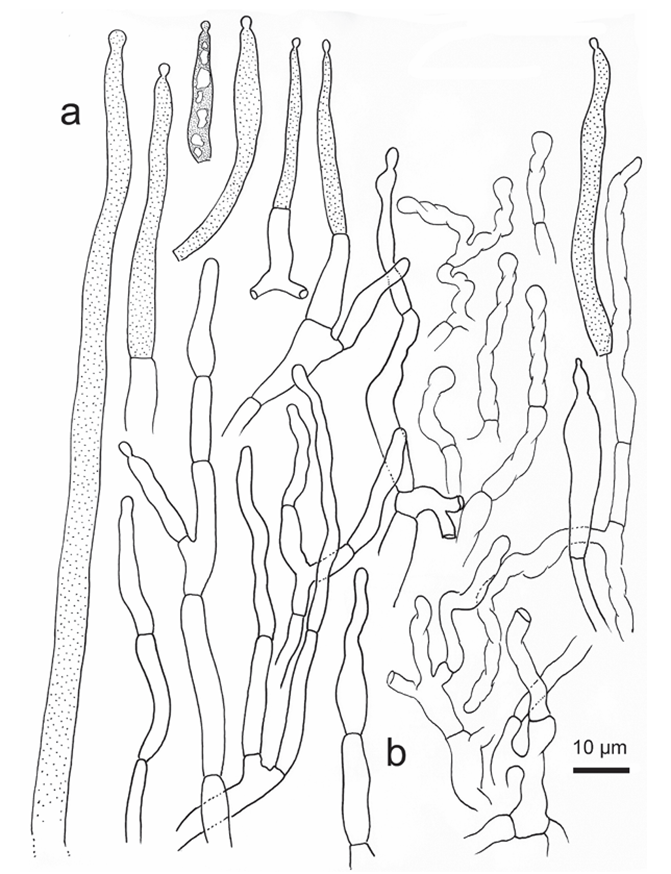
Figure 11. Russula succinea, holotype A hyphal ends in pileipellis margin B hyphal ends in pileus centre.